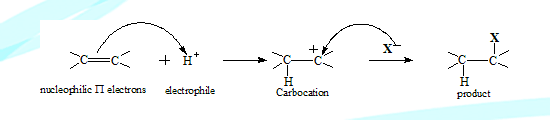
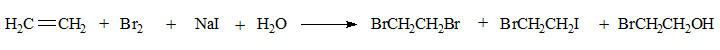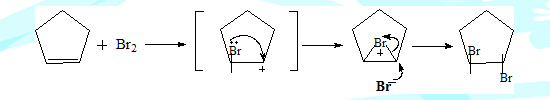The pi electrons of the multiple bonds between carbon atoms facilitate easy addition of electrophiles. The natural attraction between the nucleophilic pi electrons and the electrophiles facilitate the process..
The electrophile H+ from HX adds on to the alkenes forming a carbocation, which is attacked by the nucleophile in the second step resulting in the final product
Mechanism: Electrophilic additions generally are not a single step processes, that is HX does not approach the double bond and in a single concerted step bond breaking and formation do not take place. Instead the electrophile adds on first, followed by a nucleophilic attack (by X-) resulting in the product.
The first is slow rate determining step followed by the fast step. Since an electrophile is involved in the rate determining step it is considered as an electrophilic addition.
It is designated by IUPAC as AE + AN process (first step AE and second AN)
The well known test for unsaturation for an organic compound is another example of addition reaction. Bromine adds on to the double bond forming a 1,2-dibromo compound in the process the orange colour of bromine is discharged.
This reaction also takes place in two steps. Bromine molecule by itself is not an electrophile, under the influence of the approaching reagent it polarises into slightly positive and negative ends. The pi electrons then will bond with the positive end of bromine forming a carbocation and a bromide ion. The bromide ion in the second step will bond with the carbocation resulting in the final product, 1,2-dibromoethane.
Evidence that the reaction is not a single step process
The reaction between bromine and ethene is conducted in the presence of NaI and H2O. It is found that along with the expected dibromo product (BrCH2CH2Br )two more are obtained as follows.
The Bromoiodoethane and the bromoalcohol could not have been formed from the dibromo product through simple substitution under the reaction conditions. Iodide and H2O are too weak as nucleophiles.
Also Iodide and H2O could not have added directly to ethene for the same reason. These additions can take place to alkenes but require harsher conditions.
On the other hand they could easily add on to the carbocation, thus supporting the two step process in which the carbocation is formed in the first step.
In cyclic alkenes when bromine adds on there is another stereochemical factor that needs to be considered.
Bromine adds on to cyclopentene to give trans-1,2-Dibromocyclopentane exclusively. Its cis isomer is not formed at all.
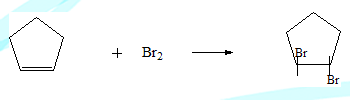
This is strange because as discussed earlier if the reaction were to go through a carbocation intermediate the cis product also should have formed. The carbon of the carbocation is sp2 hybridised and hence planar. The nucleophile can access it from both sides resulting in the two isomeric products. Clearly the intermediate involved here is different.
The bromonium ion concept: The carbocation species formed does not have sufficient life time to interact with the nucleophile. The lone pair of Bromine interacts with the adjacent positive charge to form a bromonium ion intermediate. In the next fast step the nucleophile attacks resulting in the product.Clearly in the second step the approach of the nucleophile is confined from one side of the cyclopentyl ring resulting in only the trans-isomer.The nucleophile cannot approach from the opposite side because of steric hindrance for the appraoaching nucleophile by the Bromine containing three membered ring system. The sequence is illustrated below.