
Markonikov addition (Markownikoff)
When a hydrogen halide like HBr adds on to a symmetrical alkene like ethene there is only one product possible, whether the hydrogen of HBr adds on to the first or the next carbon.
The situation is different when the addition is to an unsymmetrical alkene like propene or 1-Butene. There are two possible products.
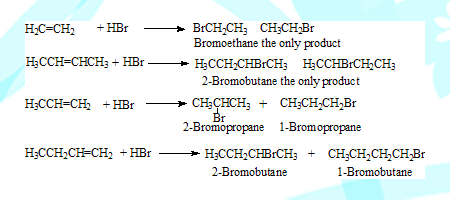
Out of the two possibilities only one is almost exclusively formed. This can be found out by using “Markonikov rlue” which can be stated in two forms.
“When hydrogen halide adds on to unsymmetrical alkene the hydrogen always adds on to the least substituted carbon.” ( the carbon which has most hydrogens attached to it)
Addition takes place at the double bond so only the two carbons linked by the double bond are considered for applying the rule. In propene the CH2 carbon is least substituted so hydrogen adds on to it and bromine to the other leading to 2-Bromopropane. Similarly in 1-Butene CH2 carbon is least substituted leading to 2-Bromobutane.
Modern statement
Addition to an unsymmetrical alkene takes place so that the positive part of the addendum adds on to such a carbon so that the reaction goes through a more stable carbocation.
Addition of HX to an alkene takes place in two steps in the first step the electrophile adds on forming a carbocation which undergoes nucleophilic attack resulting in the product.
The electrophile can add on to either of the two carbons connected by the double bond. This can form two different carbocations, but the stable one predominates.
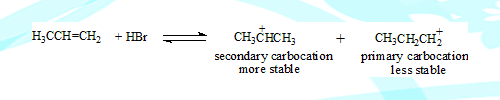
In the second and fast step the nucleophile and the more stable carbocation form the final product.

Anti-Markonikov addition
Under some conditions the opposite of markonikov addition known as anti-markonikov addition takes place. Generally this is addition of HBr in presence of organic peroxide to an unsymmetrical alkene. This is called peroxide effect.

In the presence of peroxide the reaction mechanism changes from ionic to free radical process. The peroxide undergoes cleavage under thermal or photochemical conditions to form a radical.
The radical thus formed from peroxide abstracts a hydrogen atom from the addendum (HBr) forming a bromine radical. This in turn adds on to the double bond in such a way it goes through a more stable intermediate leading to 1-Bromopropane the anti markonikov product.
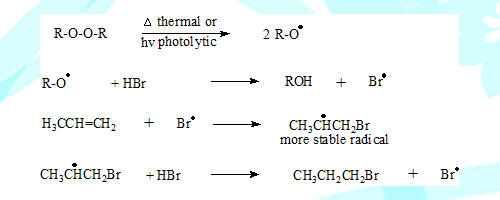
Note: Addition of HBr in the absence of peroxide will give the normal Markonikov product.
In some additions anti-Markonikov product is formed even through ionic mechanisms. The reason again is the process goes through the more stable intermediate. To solve such problems, identify both possible intermediates and continue using the more stable one.
Examples
CH2 =CH-N+ Me3I- + HI
CH2=CHCOOH + HCl
Nucleophilic addition to the double bond
Normally a nucleophile will not add on to the double bond because it will be repelled by the pi electrons. Addition is possible when an electron withdrawing group is present adjacent to the double bond, as in the case of alpha beta unsaturated carbonyl compound, which is also termed Michael addition.
Some electron withdrawing groups : -CHO, -COR, -COOR, -CONH2, -CN, -NO2,
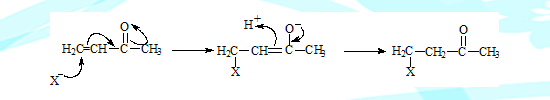
The oxygen with the negative charge can get protonated, however the enol formed easily undergoes tautomerisation forming the more stale carbonyl compound.