
Sigmatropic rearangements
The FMO theory
Ms. Anita
- Sigmatropic rearrangements are a type of pericyclic reactions that go through a cyclic transition state.
- It involves migration of a sigma bond next to a π system to a new position in the molecule.
- A sigmatropic shift involves shifting of an atom or a group to a new position in the molecule.
For illustrations of a 1,3-shift, a 1,5-shift, suprafacial and antarafacial shifts,[3,3]-shifts, Cope and Claisen rearrangements please see
"Sigmatropic rearrangementsHuckel-Mobius theory"
The analysis of sigmatropic shifts and rearrangements can be easily understood by using FMO tjheory. (Frontier Molecular Orbital theory). So it is necessary to have a clear idea of Molecular orbitals of some systems and the FMO involved.
We will consider three molecules, 1,3-Butadiene, Pentadienyl radical and allyl radical, their MOs and their HOMO and the LUMO.
1,3-Butadiene.
The four p-orbitals of the molecule undergo linear combination of atomic orbitals ( LCAO)and form four molecular orbitals out of whiich two are of lower energy and two of higher energy than the original p-atomic orbitals. The energy profile and the Mos with phase signs are shown below.
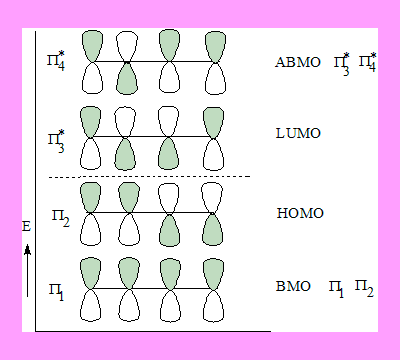
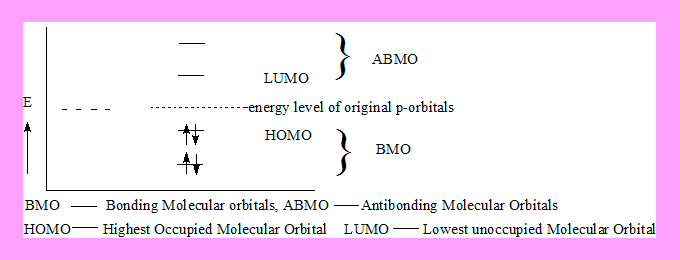
The four π electrons of Butdiene go into π1 and π2 , they are the occupied levels. Out of this π2 is of higher energy hence termed
Highest occupied Molecular orbital ( HOMO ).
π3* and π4* are of higher energy they are anitbonding in nature. Out of this π3* is of lower energy hence termed Lowest Unoccupied Molecular orbital.(LUMO). HOMO and LUMO are the Frontier molecular orbitals because they are on either side of the original energy level of π atomic orbitals.
The principle of the theory is that reaction between two moleucles involves interaction between the HOMO of one and the LUMO of the other. Within a single moleule when energy is supplied it is the electron from HOMO that undergoes tranisition to the LUMO.
The phase signs of HOMO becomes important in any discussion of application of FMO theory. The shaded lobe may be conidered to be the +ve phase sign and the blank the -ve.