
Sigmatropic rearangements
Analysis using FMO theory
Ms. Anita
- In the analysis using FMO method:
- The C-H bond that is migrating is assumed to be homolytically split.
- The resulting carbon free radical and the orbital of the hydrogen atom are involved in the reaction.
- The HOMO of the carbon free radical and the hydrogen orbital are interacting in the transition state.
1,3-shift: FMO of allyl radical and H atom

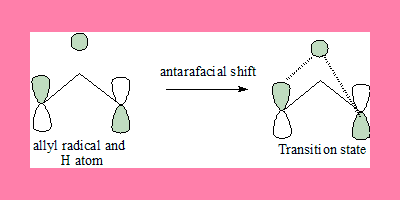
In this 1,3-suprafacial shift transition state, the interaction has a nodal plane hence the process is forbidden under thermal conditions.Under photochemical conditioons because of absence of nodal plane it is allowed but geometrically it cannot take place.
1,3-shift: Under photochemical conditions
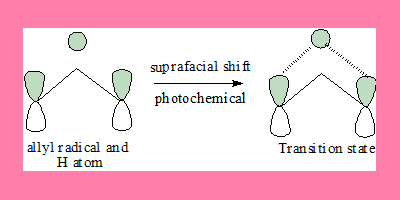
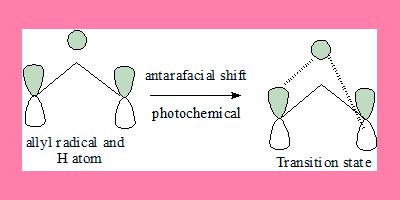
In this 1,3-suprafacial shift transition state, the interaction has a no nodal plane hence the process is allowed under photochemical geometrically also it can take place. Note: the FMO under photochemical conditions is π3*.
1,5-shift: FMO of pentadienyl radical and H atom
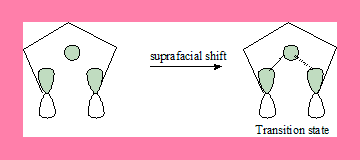
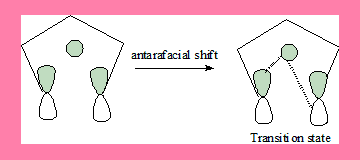
In this 1,5-suprafacial shift transition state, the interaction has a no nodal plane hence the process is allowed under thermal conditions.Geometrically also it can take place. In the antarafacial shift there is a nodal plane hence it is a forbidden process, and geometrically it is not feasible.
1,5-shift: Under photochemical conditions
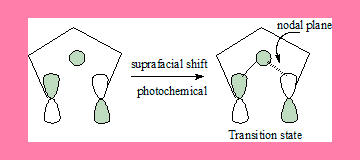
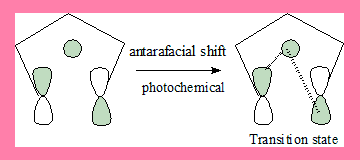
In this 1,5-suprafacial shift transition state under photochemical conditions, the interaction has a nodal plane hence the process is forbidden. Geometrically also it is not feasible. In the antarafacial shift there is no nodal plane hence it is an allowed process.
Copyrights: 2005 www.chemvista.org All Rights Reserved.
Author Ms. Anita.