
- Free rotation is possible about the carbon-carbon single bond axis.
- The rotation makes the atoms in a molecule assume different spatial positions with respect to each other.
- The different spatial positions are called "conformations".
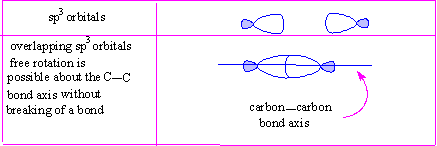
Rotation about the C-C single bond
- Looking from the front Hb is eclipsed by Ha the angle between the plane containing Ha and Hb is zero (they are in the same plane)
- The second conformation has Hb at a different spatial position, they are not in the same plane. The plane containing Hb is 600 to the right of the plane containing Ha. This is the dihedral angle, in the first case the dihedral angle is zero.
- In the third case though the two atoms are in the same plane Hb has undergone a rotation of 1800 and that is the dihedral angle.
- The dihedral angle is used when two atoms are separated by three bonds.
In a simple molecule like ethane, the conformation in which the atoms are closest to each other is called the eclipsed form, and the one in which they are farthest apart is the staggered conformation. Inter atomic repulsions are maximum in the eclipsed and minimum in the staggered form thereby making the former less stable than the later.
Representation of the 3D structures of conformations for simple molecules.
The Newmann projection
Ethane has two carbons and three hydrogens attached to each carbon, the two carbons are joined by a single bond about which rotation is possible. First a circle is drawn and the center of the circle represents the carbon in front when you are looking at the molecule (see the diagram). That carbon has three hydrogens attached, the carbon in the back also has three hydrogens attached but they are not visible because they are exactly behind the ones in front looking in that specific direction, this is the eclipsed form. In the diagram for the carbon in front the bonds start in the middle of the circle and the one in the back they are drawn starting from the edge of the circle. In the eclipsed form the hydrogens in the back are drawn with a dotted line close to the one in front indicating they are eclipsed.
Conformationsof ethane "The Sawhorse form"
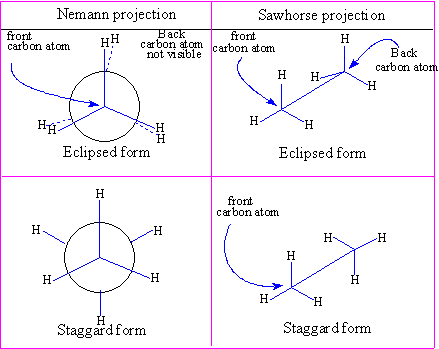
- Very little energy is needed for conformational changes, so they will be taking place at all temperatures, less at lower temperatures. For instance the eclipsed form will be converting to the staggered form and vice-versa. So the two forms are in equilibrium with each other with more stable one predominating.
- At any given temperaturethe % of each form
is fixed. This can be calculated by using the Gibbs equation
? ΔG0 = -2.303RT log K
(R = universal gas constant, T = temp in k and K = equlibrium constant= x/100-x, where x represents the % of one form, ΔG0 = free energy difference between the two conformers.) - Conversion of one form to the other generally requires 1800 rotation about the bond axis keeping one carbon fixed, except in simple cases.
- For further information: Organic Chemistry, Graham Solomon or Wade. At a higher level: Stereochemistry Eliel or Nasipuri.