
Cycloaddition reactions
- There are certain reactions which take place through a cyclic transition state called Pericyclic reactions
- These reactions generally are not influenced by a solvent or a catalyst.
- They are Electrocyclic reactions, Sigmatropic rearrangements, Cheleotropic reactions and Cycloadditions.
- The feasibility of these reactions can be ascertained by three methods.
- 1. By applying te Woodward-Hoffmann symmetry correlation of the molecular orbitals involved in the process
- 2. By using the PMO method ( Huckel-Mobius considerations)
- 3. By the FMO (Frontier Molecular Orbitals method).
- In this article the FMO method is applied for [2+2] cyclo addition reactions and the possibility of the reaction under thermal and photo chemical conditions is determined.
- The numbers denote the number of electrons involved in each of the molecules.
A nodal plane is a plane of zero electron density.
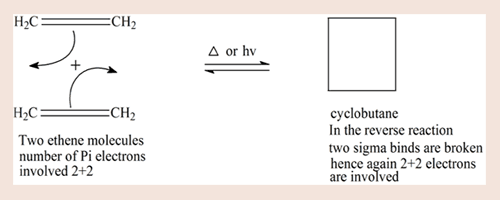
The following reaction between two ethene molecules leading to cyclobutane, is a [2+2]- cycloaddition
The π-Molecular orbital picture of ethene is given below.
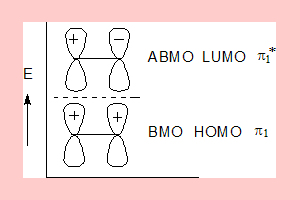
- In the above π- Molecular Orbital (π-MO) picture of ethene:
- π1 is the Bonding and the Highest Occupied Molecular Orbital( BMO and HOMO).
- π1* is the Anti-Bonding and the Lowest Unoccupied Molecular Orbital.(ABMO and LUMO)
- The two π electrons in ethene will occupy the lowest energy level which is π1 and π1* is unoccupied.
- Under photochemical conditions an electron undergoes excitation to the next level so π1* will then be the HOMO.
- The lowest energy MO π1 has zero nodal planes and the highest energy MO π1* has one nodal plane.
The FMO theory.
- This theory considers only the Frontier MOs, the HOMO of one ethene molecule and the LUMO of another.
- Only if the interacting lobes are in phase the reaction is thermally feasible.
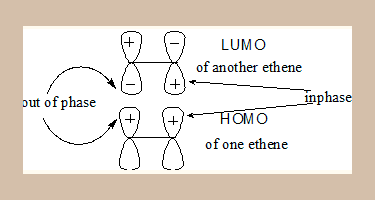
- In the MO diagram the lobes on both sides are not in phase, hence this process is thermally not feasible.
- On photo-irradiation one of the electrons from π1 under goes excitation to π1*. Thus under photochemical conditions the HOMO is π1*.
- The FMO picture is:
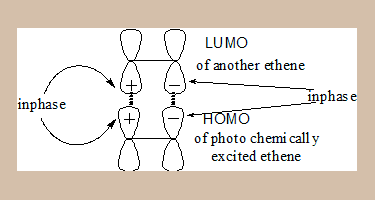
In the MO diagram the lobes on both sides are in phase, hence this process is photochemically feasible.
Copyrights: 2005 www.chemvista.org All Rights Reserved.
Author Ms. Savitha.K.T.