

- Daniel Shechtman: Israeli citizen. Born 1941 in Tel Aviv, Israel.
- Ph.D. 1972 from Technion -- Israel Institute of Technology, Haifa, Israel.
- Distinguished Professor, The Philip Tobias Chair, Technion -- Israel Institute of Technology, Haifa, Israel.
- http://www.guardian.co.uk/science/2011/oct/05/nobel-prize-chemistry-work-quasicrystals(source of the picture)
- Nobel prize in Chemistry 2011
Solids are generally classified as crystalline or amorphous.
Crystalline solids have atoms or ions arranged in a definite pattern. The basic arrangement is called a unit cell which keeps repeating itself along the three axes to form the bulk of the matter.
For instance the unit cell in certain solids is a simple cube. It has 8 corners with atoms at each point this arrangement extends in all directions (along the three axes). Each atom will have 6 immediate neighbours and it is called the co-ordination number.
Amorphous solids do not have a repeating pattern as in the case of glass.
Before 1982 it was believed that every crystalline solid should have an arrangement which is symmetrical with the unit cell extending in three dimensions.
From the point of view of classical crystallography a crystal can possess only two, three, four, and six-fold rotational symmetries.
The Israeli scientist, Daniel Shechtman and his group through electron microscopy and diffraction pattern studies clearly demonstrated a diffraction pattern with fivefold symmetry. The pattern was recorded from an Al-Mn alloy which had been rapidly cooled after melting. This historic observation led to startling conclusions which were unthinkable at that time.
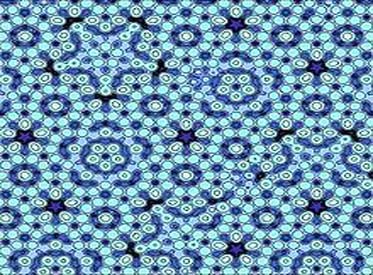
Atomic model of an aluminum-palladium-manganese (Al-Pd-Mn) quasicrystal surface.
http://en.wikipedia.org/wiki/Quasicrystal(image source)