In this page
Streochemistry
Structural isomers
Positional isomers
Optical activity
Stereochemistry
Chemistry that deals with arrangement of atoms and groups in space and the consequent differences in properties.
Stereo isomers
Isomers that have the same molecular formula same structure but different spatial arrangement of groups.
Configuration
The spatial arrangements of atoms or groups in a molecule for instance around a stereo centre.
Structural isomers:
Have the same molecular formula but different structure. They are also called constitutional isomers.(They are not stereo isomers)
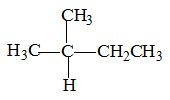
Chain isomers:
One of the three types of Structural isomers, the other two are Functional and Positional isomers. They have same molecular formula but different chain length carbon frame work.
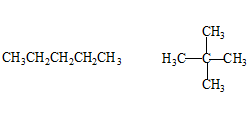
One more chain isomer with the same molecular formula is possible, shown on the right.
Functional isomers:
Same molecular formula but different functional group
1. Methoxymethane and Ethanol CH3-O-CH3 and CH3-CH2OH. 2. Propanal and Propanone(Acetone)
CH3CH2CHO and CH3COCH3
Positional isomers:
Same molecular formula but different position of substituent or functional group
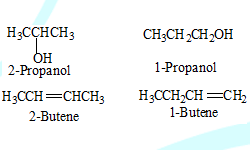
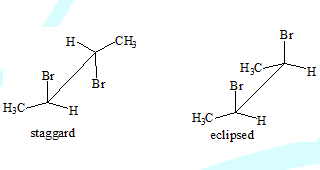
Conformational isomers
A type of Stereo isomers.( cis,trans and optical isomers are the other types)
The different spatial arrangement of atoms or groups that result due to rotation about carbon-carbon single bond in a molecule.
Between the two extremes there are infinite number of conformations possible, termed gauche forms.
Geometric isomers (cis-trans isomers)
The different spatial arrangements that exist due to restricted rotation like in molecules that have double bonds or ring systems as in cyclohexane.
Cis-trans isomerism is possible in alkenes only when the two groups attached to each of the sp2 carbons linked by a double bond are different.
In IUPAC system they are desinated as E and Z isomers. In many cases E corresponds to trans and Z to cis. In cyclic molecules if two groups are on the same side of the ring it is cis, if they are on the opposite side it is trans.
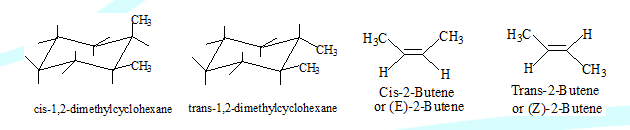
Optical activity
It is the property of a molecule to rotate the plane of polarisation of plane polarised light. This can be detected / measured by using an instrument called polarimeter.
Optical isomers/Enantiomers:
Molecules without elements of symmetry are optically active. They exist as a pair of non superimposable mirror images of each other. If one rotates the plane of plane polarised light to the left the other will do so to the right, but the magnitude of rotation is the same. Such a pair of isomers is optical isomers, also termed enantiomers.
Diastreomers
are stereo isomers which are not mirror images of each other. Examples cis-trans isomers
isomers of tartaric acid that is meso tartaric acid and (+) or (-)-tartaric acid.
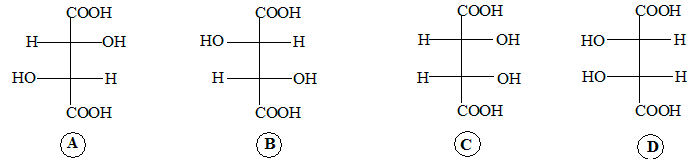
A and B are stereo isomers which are mirror images of each other they are a pair of enantiomers.
C and D are identical, they have a plane of symmetry. A and C are stereo isomers but not mirror images of each other,they are a pair of diastereomers. So are B and C.