Isomerism
Structural Isomersim Exercise:
1 |
The number of structural isomeric alcohols of
the molecular formula
C4 H10 O are: |
a) 4 |
b) 3 |
c) 2 |
d) 16 |
2 |
Which of the following is a chain isomer of 2,2-Dimethylpropane? |
a) 2-Methylpentane |
b) Pentane |
c) 2,3-Dimethylbutane |
d) all of
them
|
3 |
Which one of the following are functional isomers of 1-Butanol? |
a) Methoxypropane |
b) Ethoxyethane |
c) 2-Butanol |
d) both a and b |
4 |
Which one of the following are not a pair of positional isomers? |
a) 2-Pentene and 1-Pentene |
b) 5-Chloro-1-pentene & 5-Chloro-2-pentene |
c) 2-Chloropentane & 3-Chloropentane |
d) 3-Chloropentane & 2-Chlorohexane |
5 |
2-Butanol and Ethoxyethane are a pair of: |
a) positional isomers |
b) chain isomers |
c) functional isomers |
d)stereoisomers |
6 |
How many constitutional isomers are possible with the molecular
formula C3H5 Br3? |
a) 6) |
b) 4 |
c) 5 |
d) 8 |
7 |
How many positional isomers are possible for trimethyl benzene? |
a) 2) |
b) 3 |
c) 4 |
d) 5 |
8 |
The number of chain isomers of a hydrocarbon with molecular
formula C5H12 are 3, what will be the
number for C7 H16? |
a) 5 |
b) 8 |
c) 7 |
d) more than 8 |
9 |
The number of structural isomers formed on mono-chlorination
of 2-Methylbutane are: |
a) 2) |
b) 3 |
c) 4 |
d) 5 |
10 |
1-Butanol is not a structural isomer of: |
a) 2-Butanol |
b) 2-Methyl-2-propanol |
c) 2-Methylpropanol |
d) Cyclobutanol |
11 |
Isomers which are a result of free rotation of carbon-carbon
bond axis are: |
a) Positional isomers |
b) chain isomers |
c) functional isomers |
d) conformational isomers |
12 |
Metamerism is another type of structural isomers, they ave
the same molecular formula, the same functional group but different
carbon frame work.Which of the following are a pair of metamers?
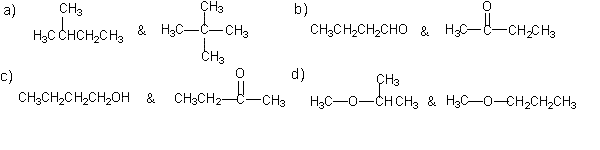
|
13 |
Structural isomers( Constitutional isomers) have the same
molecular formula but different: |
a) carbon chain |
b) functional groups |
c) structure |
d) all of them |
14 |
The compounds CH3CH=CHCH3 and CH3CH2CH=CH3 are: |
a) functional isomers |
b) chain isomers |
c) metamers |
d) have the same number
of sp3 and sp2 carbons |
15 |
How many minimum number of carbon atoms are necessary in
an alkane for it to exist in two chain isomeric forms? |
a) 2 |
b) 3 |
c) 4 |
d) 5 |
Answers:
1.b, 2.b, 3.d, 4.d, 5.c, 6.b, 7.b, 8.d, 9.c, 10.d, 11.
d, 12. d, 13. c, 14.
d, 15. c |