Iodoform testA compound containing one of the following structural units on heating with I2 and NaOH will give a yellow precipitate of Iodoform ( CHI3). This is called Iodoform test.
The reaction is used as a diagnostic test for the presence of the groups shown below.
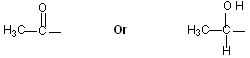
Applications:
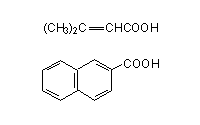
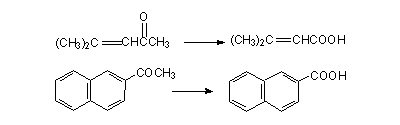
Apart from using as a diagnostic test it can also be used to prepare certain carboxylic acids with considerable ease.
Exercise
1. Give the structure of compounds which give a positive Iodoform test and have the following characterisitics.
a) An aldehyde.
b) Two ketones.
c) An aromatic ketone.
d) An alcohol with four carbon atoms.
2). Give the structure ofcompounds which do not give positive Iodoform testand have the following characteristics.
a) An aldegyde
b) An aromatic ketone
c) A cyclic ketone
3). Give reasons:
a) 3-Pentanone has a methyl and a carbonyl group, still does not give a positive Iodoform test.
c) 1-Propanol does not give a positive Iodoform test.
d) Formation of Iodoform can be used as a test to detect a methyl ketone while formation of chloroform cannot be used.
4). Write the equations for the preparation of the following carboxylic acids through haloform reaction.