
Types of electronic transitions
- When UV radiation is incident on a sample it causes electronic excitation.
- There are basically six different types of excitations requiring varying amounts of energy. Detection of these transitions their energy or the corresponding wave length can give valuable information about the sample.
- The sigma (σ) the Pi (Π) and the "n" valence electrons (non bonded electrons or lone pairs) can be present in different molecular orbitals in the ground state of an organic molecule.
- Through absorption of energy they can undergo transition to higher energy levels. The various possible electronic transitions are shown in the following scheme. Though there are six possible transitions the most useful are Π--->Π* and the n--->Π* transitions, the later has a low probability of transition which is reflected in its low ε value which is generally of the order of a few hundreds or less. The ε value for a Π--->Π* is very high of the order of a few thousands.
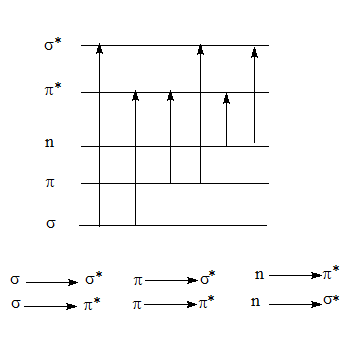
Conjugated systems
- When conjugation (presence of alternate double bonds) increases, the energy difference between the HOMO (highest occupied molecular orbital) and LUMO (lowest unoccupied molecular orbital) decreases, less energy is required for electronic transition and thus λmax of absorption increases. Comparison of λmaxcan be used for identification of some structural differences or similarities in molecules.
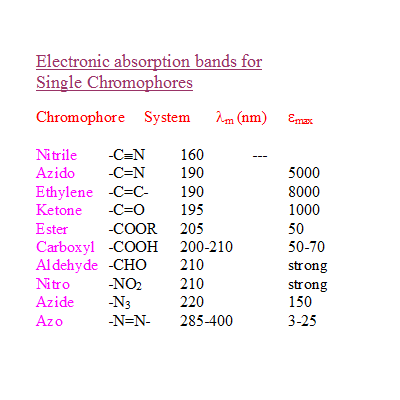
Specific chromophores
A chromophore is a light absorbing group in the UV Visible range which usually has a Pi (Π)bond.
An auxochrome by itself does not absorb light in that range but when present in conjugation with a chomophore will enhance the intensity and the position of the λmax. It usually has an atom with one or more lone pairs of electrons. Tthe λmax is indication of some groups.
C=C 171 nm (15,500)
Auxochromes: -OH, -NH2, -OR,
(C=C)n n = 2 217 nm (21,000), n = 6 364 nm (138,000)
Benzene 254 nm (169) and 204 nm (7,500)
C=O 279 nm (15) due to n--->Π * and 189 nm (900) due to Π--->Π*
C=C-C=O 314 nm (38) due to n---> Π* and 224 nm (9750) due to Π--->Π*
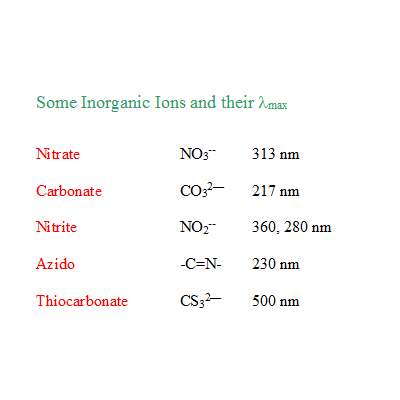
1. Compare the λmax of NO3— and NO2—
a) Rationalise why nitrite has two absorption maxima?
b) Which of them is due to n--->Π* transition?
c) Account for the lower λmax 280 nm of nitrite in comparison with 330 nm of nitrate.