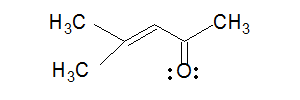
C=C
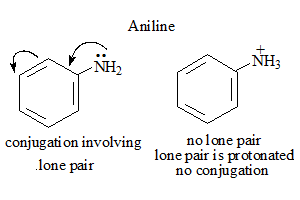
A double bond is non polar in the ground state and polar in the excited state. A change of solvent from non-polar to polar will therefore stabilize the excited state that is less energy is required for such a transition. Hence there will be increase in the λmax Increase in λmax is termed Bathochromic shift or red shift. and decrease as Hypsochromic shift (blue shift).
C=O:
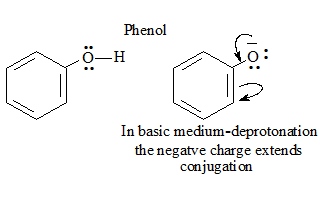
A double bond having an atom with a lone pair is polar in the ground state, a polar solvent will stabilize the ground state more than the excited state. Higher energy is necessary for n--->Π* transition, when solvent is changed from non-polar to polar, λmax will decrease. and decrease is termed as Hypsochromic shift or blue shift.
n--->Π* blue shift of 25 nm Hypsochromic shift
Π--->Π* red shift of 13 nm Bathochromic shift
Compound : Aniline
Compound :Phenol
- In basic medium deprotonation occurs in phenol thus increasing the conjugation and influencing the absorption.