
Asymmetric Synthesis -Addition of HCN to an aldehyde
Addition of HCN to acetaldehyde to form a cyanodydrin, is a well known reaction. The cyanide anion from HCN as a nucleophile attacks the electrophilic centre of the carbonyl carbon of the aldehyde.
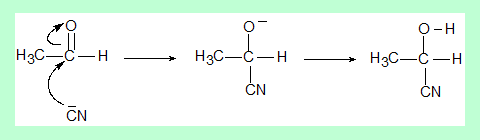
- The sp2 carbonyl carbon is planar, that is, the atoms bonded to it and itself are in one plane.
- So the nucleophile can approach from front or back, each resulting in a different configuration (R or S)
- Thereby resulting in racemic mixture as the product.
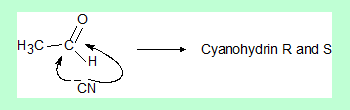
- The probability of the nucleophile attacking the electrophilic centre in the above case from either side is almost equal.
- If the nucleophile attacks from one direction predominantly it will result in one of the enantiomers as the major product.
This is the basis of “enantioselective synthesis” or “asymmetric synthesis”. - R and S, imply the configuration of the stereocentre and not necccesarily concerned with optical rotation.
- So a compound with stereocentre R may be dextro or laevo rotatory.
- And in a racemic mixture if one has R configuration the other has S and if one of them is dextro the other is laevo rotatory.
Enantiomers have the same physical and chemical properties, they differ in two ways.
- The optical properties are different: one of them rotates the plane of polarisation of plane polarised light to the right it is dextro rotatory, the symbol is (+) or (d) and the other to the left (-) or (l). The magnitude of rotation however is the same.
- When an optically inactive that is an achiral molecule approaches a chiral molecule the interaction is the same with either enantiomer.
- When a chiral molecule approaches another chiral molecule the interaction is different.
- For instance, (-) Ephedrine is pharmacologically active and naturally occurring ( control of asthama ) and its enantiomer is inactive. Sometimes an enantiomer can be poisonous while the other beneficial. Why is it so?
- The spatial arrangement of groups (configuration)around a stereo centre is diiferent. Thus when one enantiomer approaches a chiral system (like an enzyme) its configuration matches producing the desired effect. The configuration of the other enentiomer does not match.
- Because of difference in phyiologically activity of each enantiomer, it may be necessary to get hold of each one, in pure form.
- There are two ways of achieving this, separate them from a racemic mixture, or synthesise the desired one in the pure form.
- Both are not easy.
- Asymmetric synthesis is trying to synthesise one of the enantiomers exclusively.
Copyrights: 2005 www.chemvista.org All Rights Reserved
The image in the header section is from Wikipedia
The image in the header section is from Wikipedia