
Asymmetric Synthesis -Transition state Energies
Enantioselectivity-Basic Principles
By use of certain catalysts and suitable reaction condions it is possible to make one of the enantiomers as the major/exclusive product. This is enantioselective synthesis
- Generally in Organic synthesis a combination of a nucleophile with an electrophilic centre of the substrate occurs. The approaching nucleophile can reach the electrophlic centre from two different directions each leading to a particular enantiomer.
- So if that approach is blocked from one direction it should and does result in a specific enantiomer.
- This is generally achieved by using a chiral catalyst or solvent.
- Eenantioselectivity can also be explained on the basis of transition state energies. An example is of a reduction with chiral hydride donors.
- How enantioselectivity takes place.
- Let us consider the reduction of acetophenone by a metal hydride leading to an alcohol which has a stereo centre hence optically active. It can have a configuration either R or S.
- Acetophenone has two enantiotopic faces Re and Si.
- During reduction an organometallic hydride can approach the trigonal carbon from either side.
- If the ligand (L) from the reducing agent is not chiral the two transition states are non superimposable mirror images of each other and are of equal energy thus leading to equal amounts of R and S alcohols(racemic mixture).
- No enantioselectivity takes place
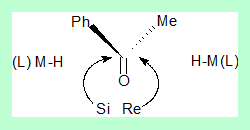
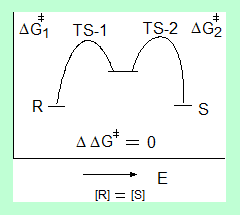
The diagram shows the two transition states for the formation of the R and S isomers and the transition state energies are same that is the difference in energies for the two pathways is zero.
No enantioselectivity.
The enantiomers are formed in about equal amounts.
Copyrights: 2005 www.chemvista.org All Rights Reserved
The image in the header section is from Wikipedia