
Cyclo Addition Reactions and Orbital symmetry Considerations A [2+2] system
By
- A Cyclo addition reaction involves the addition of two unsaturated molecules to yield a cyclic product
- A 4n system has four electrons involved in the conversion. (n is any integer, here it is 1).
- When two ethene molecules add on the product is cyclobutane, here 4π electrons are involved from two ethene molecules hence it is a 4n system or it is a [2+2] cyclo addition. It is also referred to as [ π2s + π2s]-cycloaddition. "s" indicates that it is a suprafacial process. That is the end lobes of both molecules add on from their same side with the lobes being in the same plane.
- The following diagram illustrate such a reaction.
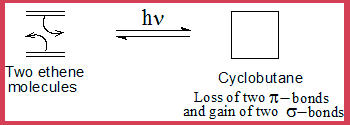
The Theory
- When two ethene molecules add on forming cyclobutane the vertical(σv) and horizontal(σh) plane of symmetry (also called mirror plane)is maintained through out the process which includes the product. (see the diagram).
- Hence the analysis is based on correlation of these symmetry elements.
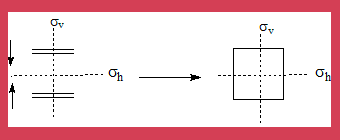
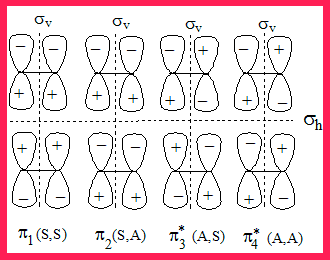
The MO diagram of the interacting orbitals and their symmetry properties
- Let us look at the MO picture of the interacting orbitals from two ethene molecules (there are four of them.
- Let us consider whether each of them has the vertical or horizontal plane of symmetry and label them as S for symmetric and A for anti-symmetric. For instance (A,S) means it is anti-symmetric in the vertical plane and is symmetric in the horizontal plane.
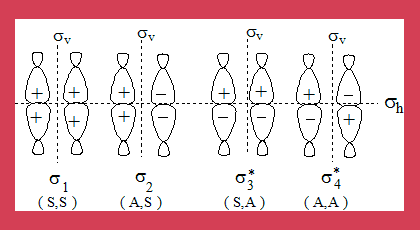
Now let us consider the interacting orbitals of the product that is, cyclobutane. Two sigma bonds are involved hence there are four interacting orbitals shown below along with their symmetry properties as discussed earlier.
Now we construct a correlation diagram using the symmetry elements of the orbitals of the reactant on one side and the product on the other.
Copyrights:2005 www.chemvista.org All Rights Reserved.