
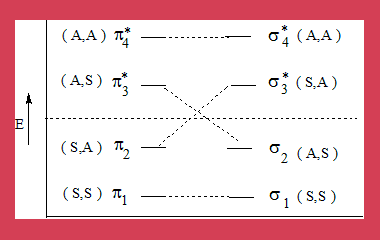
- π1 π2 are interacting bonding orbitals of ethene molecules. π3* π4* are antibonding orbitals.
- π2 is the Highest Occupied Molecular Orbital (HOMO). π3*is the Lowest Unoccupied Molecular Orbital (LUMO).
- σ1σ2are interacting bonding orbitals of cyclobutane, σ3* σ4*are its anti-bonding orbitals, σ2 is HOMO and σ3*is LUMO.
- According to Wood-Hoffmann theory, the symmetry of interacting bonding molecular orbitals of the reactants should correlate in symmetry properties with the interacting bonding molecular orbitals of the product. Similarly the antibonding orbitals of the reactant and product should correlate, if not the reaction is thermally not feasible.
- In the diagram π2 the bonding MO of reactant correlates with the anti-bonding MO σ3* of the product
- Clearly this process is thermally not feasible, that is symmetry forbidden.
- Under photochemical conditions an electron undergoes transition from the HOMO to the LUMO.
- Under such conditions the interacting MO of reactant are π1 π2and π3*and from the product they are σ1σ2and σ3*
- From the diagram it is clear that these orbitals from reactant and product do correlate. Hence a [2+2] cyclo addition is symmetry allowed under photochemical conditions.
- Here is an example of a 4n+2 system or a [4+2] cyclo addition more commonly known as a Diels-Alder reaction.
- Four π electrons from butadiene and two from ethene participate in the process.
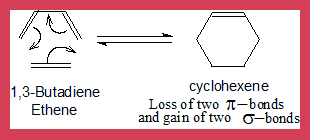
When 1,3-Butadiene and ethene molecules add on forming cyclohexene the vertical(σv) plane of symmetry (also called mirror plane)is maintained through out the process which includes the product. (see the diagram). Hence the analysis is based on correlation of this symmetry element.
The MO diagram of the interacting orbitals and their symmetry properties
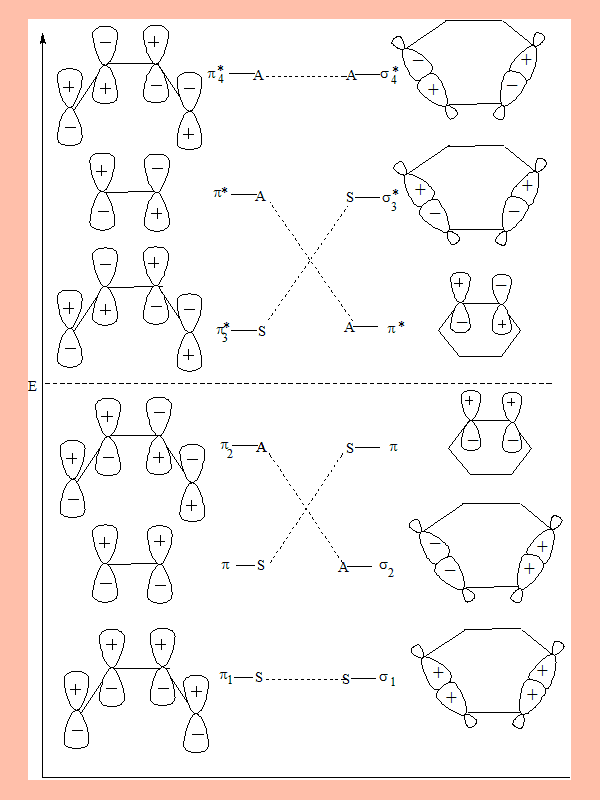
The MO with letter "S" is symmetric with respect to mirror plane, and "A" indicates anti-symmetric.
- π1 π π2 are bonding orbitals of 1,3-Butadiene and ethene. π* π3* π4* are antibonding orbitals.
- π2 is the Highest Occupied Molecular Orbital (HOMO). π3*is the Lowest Unoccupied Molecular Orbital (LUMO).
- In cyclohexene σ1σ2 π are bonding orbitals, π* σ3* σ4*are its anti-bonding orbitals, π is HOMO and π *is LUMO.
- According to Wood-Hoffmann theory, the interacting bonding molecular orbitals of the reactant should correlate in symmetry properties with the interacting bonding molecular orbitals of the product. Similarly the antibonding orbitals of the reactant and product should correlate, if not the reaction is thermally not feasible.
From the diagram: p1 p p2 the bonding MO of the reactants correlate with the bonding MO s1 s2 p of the product.
Similarly the anti-bonding MO p3* p* p4*of the reactants correlate with the antibonding MO p* s1* s4* of the product.
Clearly this process is symmetry allowed and therefore thermally feasible.
- Under photochemical conditions an electron undergoes transition from the HOMO to the LUMO.
- Under such conditions the interacting MO of reactant are π1 π π2and π3*, from the product they are σ1σ2 π π*
- From the diagram it is clear that these orbitals from reactant and product do not correlate. Hence a [4+2] cyclo addition is symmetry forbidden under photochemical conditions.