
Elimination Reactions
Consider the following reaction in which an alkyl halide is heated with alcoholic KOH:
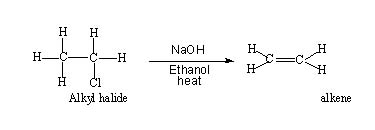
- The noticeable changes are the halogen atom and a hydrogen atom attached to the adjacent carbon are lost and a Π bond links the two carbon atoms. This is an exact reverse of what happens in an addition reaction.
- In this reaction hydrogen and a halogen atom(could be I or Br) have been eliminated hence the reaction is termed dehydrohalogenation. The two atoms or groups being eliminated are on adjacent carbon atoms, hence the process is termed beta-elimination.
It is also referred to as 1,2 - elimination. - An alkene is produced from an alkyl halide. So elimination is a method of creating a double bond.
- Instead of H and halogen other atoms are groups may be involved, for instance elimination of elements of water ( H and OH) from an alochol (dehydration)can result in an alkene. Such eliminaion reactions require a dehydrating agent.
- For elimination, the alkyl halide has to be heated with an alcoholic base which can be either NaOH or NaOC2H5 ( Sodium ethoxide in ethanol ). If aqueous base is used it results in substitution.
- The reagent NaOC2H5 is prepared by reaction of Na with excess of ethanol. The excess ethanol will serve as the solvent.
Copyrights: 2005 www.chemvista.org All Rights Reserved