Elimination Reactions
Mechanism of Elimination reactions Introduction
If you examine an elimination reaction,
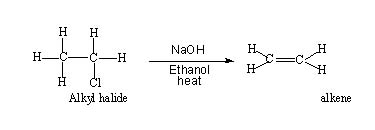
You will find that two sigma bonds are broken and a new π bond is formed. Theoretically these bond breaking and formation can take place as follows,
1. The bonds broken and formation of the π- bond can all take place in one step.
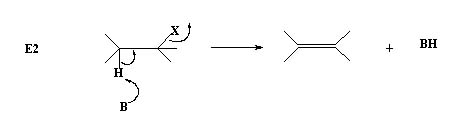
Thus the base and the alkyl halide are part of a single rate determining step hence it is a bimolecular process and is termed as E2 mechanism
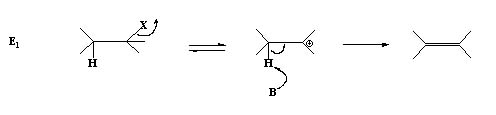
2. The carbon halogen bond may break first in a hetrolytic way resulting in a carbocation intermediate which in the subsequent step looses a proton to the base forming the alkene.
In this case the first step is the rate determining step and only one molecule is taking part hence termed as
E1 mechanism.
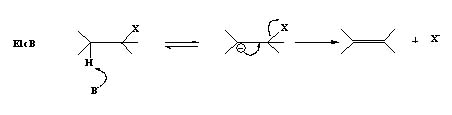
3. The Base abstracts a proton from the alkyl halide resulting in a carbanion which is the conjugate base of the alkyl halide and in the next step the negative charge forms a π-bond pushing out the leaving group.This is termed as
E1cB (E1 conjugate base).
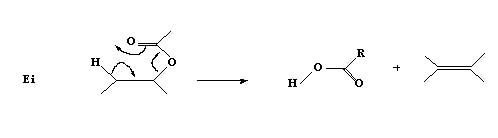
4. In some cases elimination takes place internally without a base to abstract a proton and this is termed
elimination internal Ei