E2 Mechanism Geometric requirements
For efficient overlap of the developing orbitals in cyclic systems the H and the leaving group should be anti- periplanar. In this conformation the H and the leaving group are on the opposite side of the ring and they are both in the same plane. This is possible when both of them are in axial position.
In acylic systems due to conformational rotation about the carbon-carbon bond axis this requirement is always possible but become restricted in cyclic molecules due to restricted rotation.
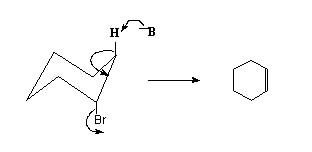
It is clear that the required anti - perplanar conformation facilitates easy elimination.
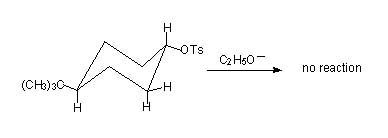
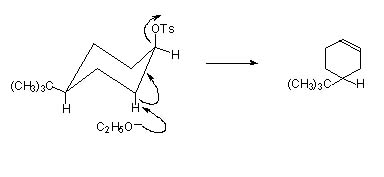
Consider two isomers of 4-tbutylcyclohexyl-p-toluenesulphonate.
In the cis isomer (-OTs and t-butyl groups ) the H and –OTs are antiperiplanar making elimination facile.
In the trans isomer this geometric requirement is not possible. Ring flip can lead to this requirement, but the bulky t-butyl group locks up the conformation so that it is always in equatorial position, hence no reaction occurs.