
Elimination Reactions
E2 Mechanism
Geometric requirements:
Another excellent example involves elimination of menthyl and neomenthyl chloride.
In Menthyl chloride the more stable conformation is the one in which the three subtituents are all equatorial, however ring flip can make these all axial(though energetically less stable) and in such a conformation elimination can occur since it meets the geometrical requirements, giving 2-menthene (even though it is the les stable product, indicating how the requirement can even over ride the stability to a certain extent).
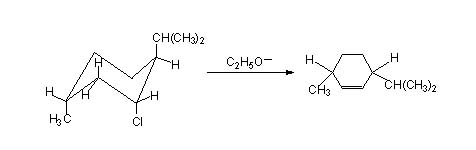
Neomenthyl chloride gives both 2-menthene and 3-menthene. This is because two hydrogens situated at carbons either side of the leaving group are available meeting the geometrical requirement.
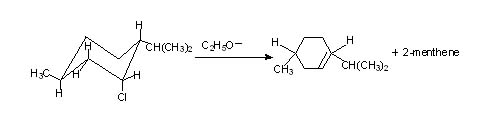
Note: In menthyl chloride elimination is slow since it has to assume the unfavourable but required conformation while in neomenthyl chloride it is fast since the more stable conformation is involved.
In menthyl chloride only one product is formed while in the other case two products are formed and the reaction is governed by Zaitsev rule.
Isopropyl group can occupy the axial position, while t-butyl group cannot due to its larger size.