
Elimination Reactions
E2 Mechanism
Influence of leaving group
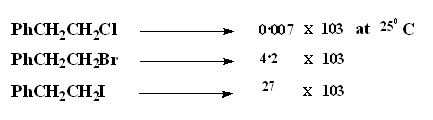
A good leaving group will favour the reaction. Since the leaving group is part of the rate determining step in an E2 process a better leaving group will increase the rate of the reaction as can be seen in the examples given above
Strength of base:
Increase in base strength increases rate: A strong base is likely to abstract a proton more easily.
-NH2 > -OEt > -OH
Isotopic labeling studies:
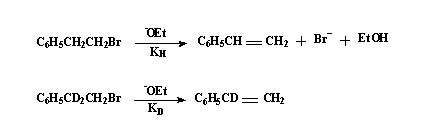
In the above equations the central carbon in the second of them has D instead of hydrogens. When both reactions are carried out under identical conditions it was found that the rate in the first case is 7.1 times greater than in the second.
That is kH / kD = 7.1
(C-H bond breaking requires lesser energy than the C-D bond). Hence the C-H bond is breaking in the rate determining step.