
Elimination Reactions
E1 Mechanism (1931 Hughes)
- Reverse of electrophilic addition
- Two step proces
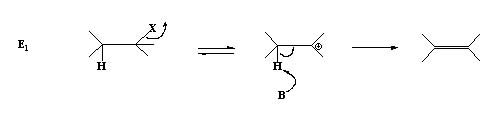
- Formation of the carbocation is the rate determining step.
R 8 [substrate] - The solvent molecules can behave as the base. No added base is required.
- A poor leaving group by protonation can made to be a good one like –OH , -NR2. Others are diazo and azide.
- Good leaving group, stability of C+, and polar solvents promote E1.
- A group with an atom having a lone pair present adjacent to the cationic center also can promote the process through stabilization of the carbocation by electron delocalisation.
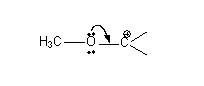
- Rearrangements are possible since C+ is the intermediate
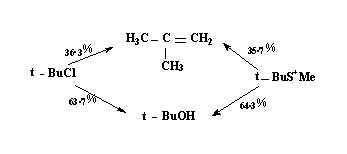
- Since the leaving group is part of the rate determining step Compounds differing in them should have differing rates of elimination. However once the carbocation intermediate is formed under similar conditions the subsequent process should be identical, which means the ratio of elimination to substitution should be same. This has been found to be the case.
Copyrights: 2005 www.chemvista.org All Rights Reserved