
Elimination Reactions
Exercise I Answers
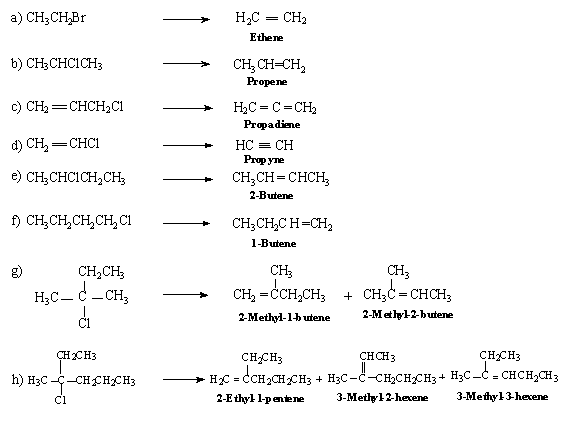
A.
- When an alkyl halide is heated with aq. KOH substitution takes place but when heated with alcoholic KOH Elimination takes place.
- The side products in an elimination reaction involving an alkyl halide and alcoholic KOH are HCl which in basic medium will be present as the salt.
- Removal of elements of water from an alcohol is also an elimination reaction, more specifically it is called dehydration, this would convert an alcohol to an alkene.
- Elimination is a method of creating a double bond in a molecule.
- Hydrogenation is addition of hydrogen across multiple bonds. Dehydrohalogenation is an elimination reaction wherein a H and a halogen is removed from adjacent atoms.
B.
Copyrights: 2005 www.chemvista.org All Rights Reserved