Movement of Pi electrons through the p-orbital pathway is Mesomeric effect and leads to resonance.
Movement of sigma electrons through the adjacent Pi system or a carbocation is Hyper conjugation. It therefore involves sigma electron delocalisation.
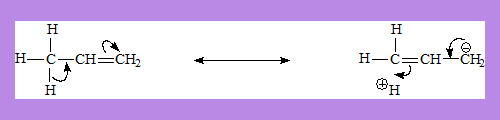
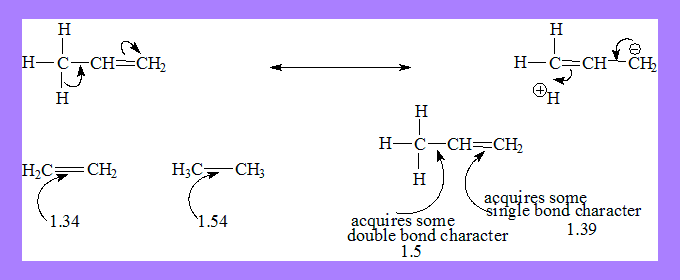
The electrons of the sigma bond between C and H are involved in delocalisation.
In the structure on the right there is no bond between a C and H due to migration of the sigma bond. Hence hyper conjugation is also called no bond resonance.
This does not mean the hydrogen atom is completely detached from the structure. It indicates some degree of ionic character in the C-H bond and some single bond character between the carbon-carbon double bond.
In Toluene there is a partial –ve charge on the carbon bonded to the methyl group and the methyl carbon is slightly +ve. This is explained by using hyper conjugation and has been proved by X-ray diffraction studies.
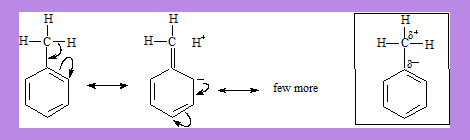
Hyper Conjugation can account for Inductive effect. In Toluene the methyl group exhibits +I effect which is responsible for the polarisation of electron density as shown
Requirements
- It exists in alkenes, arenes, carbocations and free radicals.
- The alpha carbon next to the pi bond or C+ or C free radical should be sp3 hybridised with at least one hydrogen atom bonded to it.
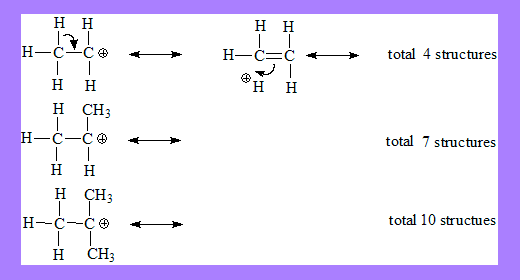
Some more examples
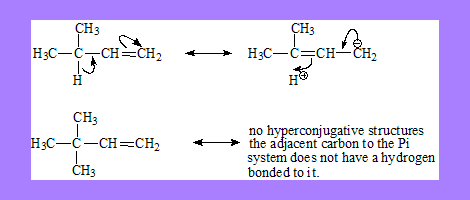
Isovalent hyper conjugation: all resonating structures have the same total charge as in carbocations and radicals.
Influence of hyper conjugation on the structure
The C-C bond acquires some double bond character and C=C acquires some single bond character.Thus C=C (double bond ) length in substituted alkenes is always greater than in ehtene
Example