Observed rotation
Specific rotation
(R) and (S) notation
D and L notation
Prochiral
Dichloromethane
Observed rotation
The rotation of plane polarized light by an optically active molecule can be measured by using an instrument called polarimeter. This is observed rotation (α)
Specific rotation
The observed rotation of a compound is not a constant and cannot be characteristic of a compound. It is dependent on concentration, temperature and wavelength. If these variables are fixed the rotation becomes constant and is called specific rotation.
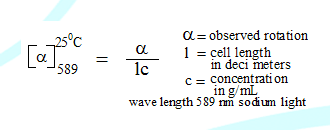
(R) and (S) notation
The configuration of enantiomers are designated using the letters R and S based on the Kahn Ingold and prelog rules.(sequences rules, or CIP rules).
The four groups around the stereo centre is given priority 1,2,3 and 4 based on the sequence rules. The lowest prority group is kept away from the direction of view and groups 1,2,3 are checked to see whether clock wise or not. If they are clockwise the configuration of the stereo centre is R if anti clockwise it is S.
D and L notation
This is a system of describing the stereo chemistry mostly in simple carbohydrates and amino acids. The configuration of the stereo centre in glyceraldehyde is assumed as shown below. (this assumption has been found to be true in later years)
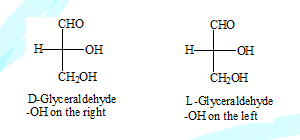
Any molecule that has the configuration similar to either one of them was considered to have the same configuration
Note: In writing there structures in Fischer projection the most oxidised carbon is written on the top and the most reduced at the bottom.
Glucose has four stereo centres but it is called as D-Glucose based on the stereo centre at the last but one carbon as in glyceraldehyde.
This system does not describe the stereochemistry of all stereo centres in a molecule.
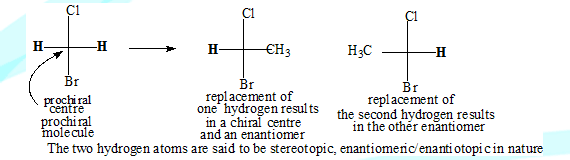
Prochiral
Other related terms: Chiral, enantiotopic, heterotopic, homotopic
If replacement of one ligand (atom or group)of an achiral centre results in a chiral centre the original molecule is said to be prochiral.
Two groups are said to be enantiotopic if substitution of each of them by the same group leads to a different enantiomer.The carbon atom in BrCH2Cl is not a stereo centre and the molecule is not chiral. If one of the hydrogen atoms is replaced by another atom the carbon will have four different groups, it will be a stereocentre the molecule will be chiral and optically active. The original molecule( BrCH2Cl) is considered as prochiral.
Replacement of one hydrogen results in one enantiomer and replacement of the other leads to the second enantiomer. The two hydrogen atoms are therefore enantiotopic in nature.
Dichloromethane
This molecule is not prochiral replacement of one of the hydrogens does not result in a chiral molecule.
The two hydrogens are not enantiotopic, they are homotopic.
Another example
One of the two hydrogens at C1 or C2 if replaced in 1,3-Propandiol with another group or atom it becomes a chiral centre
C1 and C3 are prochiral but C2 is not because it will not become a stereocentre and will still have a plane of symmetry.
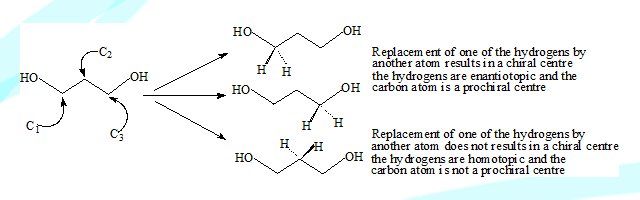
The C1(or C3) protons are topologically non equivalent since substitution of one produces a product stereochemically different from substitution of the other. Ligands (atom or group) of this type are termed heterotopic. The are also termed enantiotopic since they produce enantiomers on substitution.
The protons at C2 are homotopic they are topologically and chemically equivalent