In this page
Enantiotropic groups
Diastereotopic groups
Regio selective reactions
Re and Si faces
Chemoselective reaction
Stereoselective reactions
Stereospecific reaction
Enantiomeric excess
Asymmetric synthesis
Asymmetric induction
Enantioinduction
Asymmetric amplification
Enantiotropic groups: See “Prochiral”
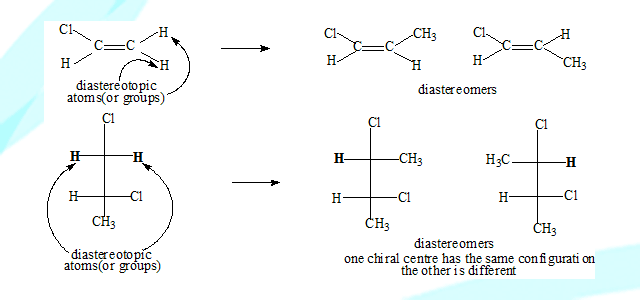
Diastereotopic groups
Two groups are said to be diastereotopic if substitution of each of them leads to a different diastereomer.
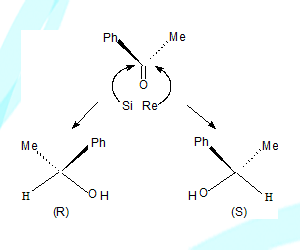
Re and Si faces
consider the molecule PhCOCH3 with carbonyl carbon as centre viewing the molecule from one side the groups O, Ph, and CH3 are in clockwise direction, this is called Re face. If the same groups are viewed from the other side they will be anti clock wise direction, this is called Se face.
On reduction the ketone can become an alcohol and the carbon concerned will be a stereo centre, Depending on the direction of approach of the nucleophile from the reducing agent say NaBH4 a specific enantiomer will result.
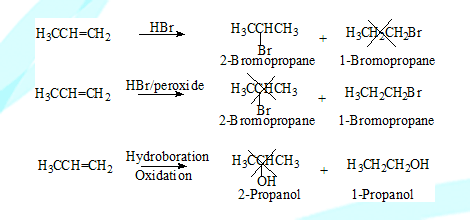
Regio selective reactions
In a reaction when there is a possibility of forming two or more structural isomers(constitutional isomers) only one is formed or is predominant, such a reaction is a region selective reaction.
Examples: Addition of HBr to an alkene in presence or absence of peroxide, Hydroboration/oxidation, Oxymercuration / demercuration.
Chemoselective reaction
A reaction in which one functional of a compound is altered leaving the other equally reactive group intact. Such a reaction is chemoselective. Obviously the reaction conditions are responsible for this selectivity
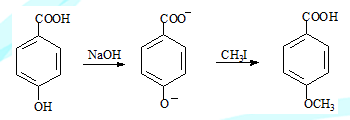
A second example
When an ester and an aldehyde is present in a compound, it is possible to reduce only the carbonyl of an aldehyde without affecting the ester by using NaBH4, LiAlH4on the other hand will reduce the carbonyl of both functional groups to the corresponding alcohols
Stereoselective reactions
Regardless of the stereochemistry of the reactants one stereoisomeric product predominates (major or exclusive)
In a stereoselective reaction it is not necessary that the reactant should have a specific stereochemistry, 2-Butyne on reduction with Ni2B (P-2) catalyst yields mainly Z-2-Butene. On reduction with Li/NH3/EtNH2 results in E-2-Butene. It is seen that the reactant is not associated with any stereochemistry but the product is one particular stereoisomer almost exclusively.
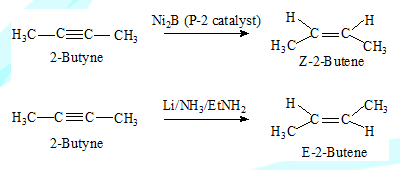
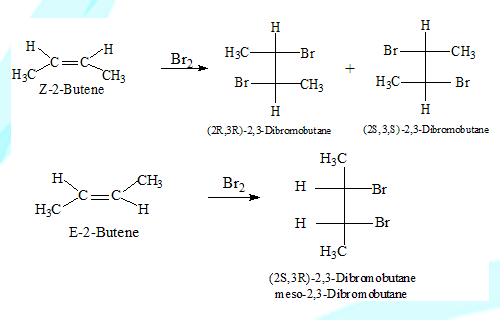
Stereospecific reaction
In a reaction one stereoisomer gives a product of certain stereochemistry while the other stereo isomer gives a product of a different stereochemistry such a reaction is stereospecific. cis-2-Butene on addition of Bromine gives (2R,3R)-1,2-dibromobutane and its enantiomer while the trans isomer gives (2R,3S)-1,2-dibromobutane(meso-1,2-dibromobutane).
Here the stereochemistry of both reactants and products is considered.
Thus a SN2 reaction is both stereospecific and stereoselective
Enantiomeric excess
When a new stereocentre is created normally both enantiomers are formed. However they need not be formed to equal extent. Their relative ratio is expressed as enantiomeric excess ( ee) or optical purity.
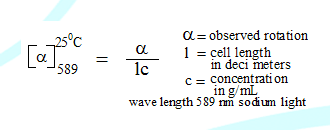
Note: Thus % of R = (ee) + % of S
If the enantiomers are formed in equal extent the mixture will be optically inactive and the enantiomeric excess will be zero. Similarly if one enantiomer is formed exclusively the enantiomeric excess will be 100.
Asymmetric synthesis
Chemical synthesis of an enantiomer, or an enantiomorphic mixture in which one enantiomer predominates. (The process should not involve resolution)
Asymmetric induction (also enantioinduction)
The formation of one enantiomer or diastereomer preferentially over the other possibilities by use of a chiral substrate, reagent, or catalyst. Asymmetric induction leads to asymmetric synthesis.
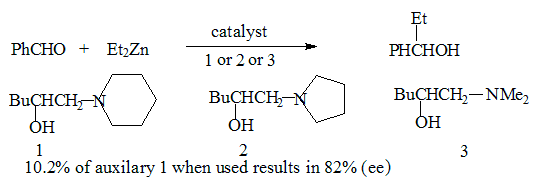
Asymmetric amplification
The enantiomeric excess (ee) of the product is generally linearly related to the ee of the auxiliary or catalyst used. In recent years, however, an increasing number of asymmetric reactions have been carried out where a small % of catalyst/auxilary group can lead to a large % of the expected product. In a reaction when the product far exceeds the amount of auxilary group or catalyst used the term asymmetric amplification is used to describe it. Theoretical models had predicted that in certain autocatalytic processes kinetically controlled asymmetric amplification,is possible the prediction has now been verified experimentally.The bidentate metal Zn, may form two types of complexes with chiral ligands. (LR, LS) two enantiomeric forms LR-M-LR and LS-M-LSand a meso form LR-M-LS. Since these are diastereomers the two types of complexes induce different rates for the formation of the two enantiomers thus bringing about asymmetric amplification.