
Hammett equation
Linear free energy relationship
Linear free energy relationship
- Substituents can have a profound influence on the dissociation of benzoic acid.
- Thus substituents can have influence on the acidity of benzoic acid.
- Hammett equation attempts to quantify this influence of substituents.
- To derive a relation only the para subtituents are considered.
- Ortho substituents complicate due to steric influence as well.
- Meta substituents are not in through resonance with the carbonyl group of benzoic acid.
- Electron withdrawing group increases dissociation while electron donating decrease dissociation of benzoic acid.
- The influence is studied at a specific temperature (250C) and a specific solvent like water.
The relation may be derived as follows,
If ΔGo is the free energy change for the dissociation of substituted benzoic acid it must be a combination of free energy change of unsubstituted benzoic acid and the influence due to the substituent which can be represented as an equation

A parameter σ is defined for each substituent in order to express the relation in more convenient form.
Thus the relation can be expressed as
The relation between free energy and equilibrium constant is
Using the above relation and substituting in the first equation.
This is the same as
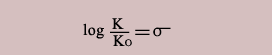
Thus substituents should influence similarly on other processes as well.
Copyrights: 2005 www.chemvista.org All Rights Reserved