
Hammett equation
Linear free energy relationship
For example
In the above dissociation though the influence of the substituent is the same but it is farther away from the reaction site. Hence the increment due to the substituent that is 2.303RT log σ which was appropriate for the dissociation of benzoic acid has to be modified by a factor ρ which is characteristic of the sensitivity of the new reaction towards electron donation or withdrawal by the substituent. Hence the earlier equation is modified including the factor ρ as,
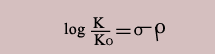
This is called as Hammett equation. It can be applied to many other equilibria and the influence of the particular substituent on the equilibrium can be predicted. Which means the direction the equilibrium shifts and the strength of the acid can be assessed. Thus it is an important equation which is useful in obtaining information about reaction mechanisms.
Hammett Plot
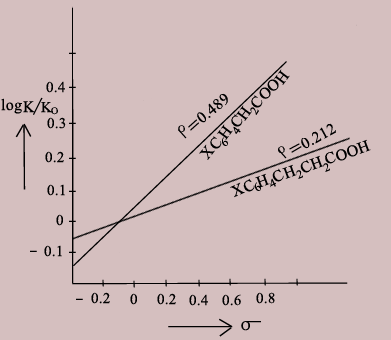
Conclusions from the Hammett Plot
- A straight line plot obtained indicates that the free energy relation of the reaction is valid.
- The slope of the line is the ρ of the reaction.
- A positive value of ρ indicates that the reaction responds to substituent effects as in benzoic acid i.e., electron withdrawing groups increase dissociation and donating groups decrease dissociation.
- If ρ > 1 then the reaction is more sensitive to the effects than in benzoic acid.
- For benzoic acid ρ is taken as 1. If the value of ρ is between 0 &1 then the effect is as in benzoic acid but lower.
- A negative value of ρ indicates that the influence of the substituent is opposite to that of benzoic acid i.e., electron withdrawing groups decrease dissociation and electron donating groups increase dissociation.
- If the value of ρ is very small, it indicates that the reaction may involve radical intermediate or a transition state.
- If the slope of the line changes abruptly it is an indication of change in mechanism as the substituents change.